CATAGORY P
Paal-Knorr Furan Synthesis
The acid-catalyzed cyclization of 1,4-dicarbonyl compounds known as the Paal-Knorr synthesis is one of the most important methods for the preparation of furans. As many methods for the synthesis of 1,4-diones have recently been developed, the synthetic utility of the Paal-Knorr reaction has improved.
Mechanism of the Paal-Knorr Furan Synthesis
A comparison of the cyclizations of meso- and dl-3,4-diethyl-2,5-hexanediones showed that these compounds cyclize at unequal rates, and that the stereochemical configuration of unchanged dione is preserved during the reaction. These findings are at odds with the commonly accepted mechanism shown here that involves the ring closure of a rapidly formed monoenol.
The rate of acid-catalyzed enolization is known not to be very sensitive to the structure of the ketone. Since the rate-determining step would be the same for both substrates, the differences in the reaction rate cannot be explained by this mechanism.
A mechanism in which the substituents would interfere differently in the rate-determining step is shown below. The ease of achieving a suitable conformation for the cyclization is not the same for both molecules:
A more detailed description can be found in the work by Amarath and Amarath, and references cited therein (J. Org. Chem., 1995, 60, 301).
====================================
Paal-Knorr Pyrrole Synthesis
The Paal-Knorr Pyrrole Synthesis is the condensation of a 1,4-dicarbonyl compound with an excess of a primary amine or ammonia to give a pyrrole.
The reaction can be conducted under neutral or weakly acidic conditions. Addition of a weak acid such as acetic acid accelerates the reaction, but the use of amine/ammonium hydrochloride salts or reactions at pH < 3 lead to furans as main products (Paal-Knorr Furan Synthesis).
Mechanism of the Paal-Knorr Pyrrole Synthesis
Amarath has shown (J. Org. Chem., 1991, 56, 6924) that meso- and dl-3,4-diethyl-2,5-hexanediones cyclize at unequal rates, and that the stereochemical configuration of the unchanged dione is preserved during the reaction. Any mechanism such as the following one that involves the formation of an enamine before the rate-determining step - the cyclization - must be ruled out.
If the ring is formed from an imine that is generated from a primary amine, a charged immonium ion must be an intermediate. Amarath tried to stabilize or destabilize the immonium ion with different aryl groups as substituents:
The use of ammonia should give an uncharged intermediate and is therefore less affected by the choice of substitutents. The substituents also influence the basicity of the imine, with the nitro group leading to a more basic nucleophile. The rates of cyclization have been compared using ammonia and methylamine. The nitro group has in every situation had a positive effect on the reaction rate. The methoxy group has a negative effect on the cyclization rate in each case. Comparison of the relative reaction rates of all substrates (R: H, Me) showed no specific stabilization/destabilization effect for a possible mechanism involving an immonium ion.
A mechanism that accounts for the influence of different substitution patterns (meso, dl) and explains the influence of a p-nitrophenyl group making a nucleophile more reactive (although not as the imine) includes the cyclization of a hemiacetal which is followed by different dehydration steps:
A more detailed description can be found in the work by Amarath, and references cited therein (J. Org. Chem., 1991, 56, 6924).
===================================
Paal-Knorr Thiophene Synthesis
Paal Thiophene Synthesis
The Paal-Knorr Thiophene Synthesis allows the generation of thiophenes by condensation of a 1,4-dicarbonyl compound in the presence of an excess of a source of sulfur such as phosphorous pentasulfide or Lawesson's reagent.
Attention: some toxic H2S is formed as a side product regardless of the sulfur source.
Mechanism of the Paal-Knorr Thiophene Synthesis
Reagents such as phosphorus pentasulfide or Lawesson's reagent act as sulfurizing agents as well as dehydrating agents, allowing a reaction pathway that could lead first to the formation of furans. This hypothesis was tested by Foye (J. Org. Chem., 1952, 17, 1405.) by treatment of different 1,4-dicarbonyl compounds and the corresponding possible furan intermediates (such as acetonylacetone and 2,5-dimethylfuran) with phosphorus pentasulfide. Using the same reaction conditions, the differences in the yields of 2,5-dimethylthiophene excludes the possibility that a predominant reaction pathway could lead through furan intermediates:Foye suggested the following reaction pathway:
Today, the occurrence of a bis-thioketone intermediate is assumed to be possible but not necessary (J. Schatz, Science of Synthesis, George Thieme Verlag Stuttgart, 2000, Vol. 9, 298.)
The reaction mechanism still needs further elucidation before it is fully understood.
====================================
Passerini Reaction

This three-component reaction between a carboxylic acid, a carbonyl compound such as a ketone or aldehyde, and an isocyanide, offers direct access to α-hydroxy carboxamides.
Mechanism of the Passerini Reaction
The Passerini Reaction proceeds rapidly if the reaction is performed in aprotic solvents at room temperature. High yields are obtained with high concentrations of the starting materials in the reaction mixture.From these findings, it is assumed that the Passerini Reaction does not follow an ionic pathway. Hydrogen bonding is believed to play a crucial role in the formation of the presumed cyclic transition state for this reaction.

=====================================
Paterno-Büchi Reaction

The photochemical [2+2] cycloaddition of a carbonyl with an olefin to give an oxetane.
Mechanism of the Paterno-Buechi Reaction
The possible transitions (C=O) are shown below:
Once the carbonyl ground state has been photoexcited, either a singlet or triplet state may be formed:
 n,π*-transition
n,π*-transitionEither type of transition (n,π* and π,π*) and electronic state (singlet, triplet) may participate in the first stage of this reaction, which is rationalized by invoking diradical intermediates:

Breaking of the new σ-bonds requires more energy, and the reverse reaction is not possible using same light frequency.
===================================
Pauson-Khand Reaction

The Pauson-Khand Reaction is a [2+2+1] cycloaddition of an alkyne, an alkene and carbon monoxide.
Mechanism of the Pauson-Khand Reaction

The following mechanism is postulated, although only the stable alkyne Co2(CO)6 complex has been isolated. (P. Magnus, Tetrahedron Lett., 1985, 26, 4851. DOI)
The stereochemistry of the complexation of the alkene at cobalt is guided by steric repulsions between the R and R' groups, so that isomers 1 and 2 are favored.

The insertion of the alkene is followed by insertion of carbon monoxide and reductive elimination of one Co unit:

Dissociation of the second Co unit gives the resulting cyclopentenone product:

====================================
Pechmann Condensation
Coumarin Synthesis
The Pechmann Condensation allows the synthesis of coumarins by reaction of phenols with β-keto esters.
Mechanism of the Pechmann Condensation
The reaction is conducted with a strong Brønstedt acid such as methanesulfonic acid or a Lewis acid such as AlCl3. The acid catalyses transesterification as well as keto-enol tautomerisation:A Michael Addition leads to the formation of the coumarin skeleton. This addition is followed by rearomatisation:
Subsequent acid-induced elimination of water gives the product:
====================================
Petasis Reaction
The Petasis Reaction is a multicomponent reaction (MCR) that enables the preparation of amines and their derivatives such as α-amino acids.
The reaction is also referred to as the Boronic Acid Mannich Reaction, since it proceeds via an imine with the organic ligand of the boronic acid acting as the nucleophile, similar to the role of the enolizable ketone component in the original Mannich Reaction.
Mechanism of the Petasis Reaction
As in the classical reaction that it resembles, the Petasis Reaction also involves a large number of interdependent equilibrium steps, some of them identical to those in the Mannich Reaction.Little is known with certainty in connection with the key step that involves the nucleophilic addition of the organic ligand from the boronate to the imine. One proposal is that the transfer is actually intramolecular, and takes place via the adduct pictured above:
Regardless of how it does take place, the fact that this addition is irreversible certainly imparts a clear advantage. In the classical Mannich, the reversibility of the final step limits the number of cases where the yields are synthetically useful. By comparison, the Boronic Acid Mannich Reaction permits a much broader scope of conversions to be carried out.
The direct reaction with glyoxylic acid merits particular mention, since it leads to interesting, unnatural α-amino acids in a single step, while avoiding the appearance of toxic byproducts such as seen with the Strecker Synthesis.
This reaction can be carried out with secondary amines, sterically hindered primary amines, hydrazines or anilines in dichloromethane at room temperature. The range of potential nucleophilic partners includes alkenylboronic acids, and electroneutral as well as electron-rich (hetero-)arylboronic acids. The conversion of electron-poor boronic acids can be effected at elevated temperatures (MW) in suitable solvents (M. Follmann et al. Synlett 2005, 1009. DOI).
====================================
Peterson Olefination

The Peterson Reaction allows the preparation of alkenes from α-silylcarbanions. The intermediate β-hydroxy silane may be isolated, and the elimination step - the Peterson Elimination - can be performed later. As the outcome of acid or base-induced elimination is different, the Peterson Olefination offers the possibility of improving the yield of the desired alkene stereoisomer by careful separation of the two diastereomeric β-hydroxy silanes and subsequently performing two different eliminations.
Mechanism of the Peterson Olefination
In the first step of the Peterson Olefination, addition of the silylcarbanion to a carbonyl compound and subsequent aqueous work up leads to diastereomeric adducts.
Some of these reactions are stereoselective and may be rationalized with simple models: The reaction of benzaldehyde and a silylcarbanion gives the threo-product if the silyl group is small. This implies that in the transition state, the two sterically demanding groups are anti. As the silyl group becomes more sterically demanding than trimethylsilyl, the selectivity shifts towards the erythro-isomer.

Acidic hydrolysis proceeds via an anti-elimination:

In contrast, the base-catalyzed elimination may proceed via a 1,3-shift of the silyl group after deprotonation, or with the formation of a pentacoordinate 1,2-oxasiletanide that subsequently undergoes cycloreversion:

The use of α-silyl organomagnesium compounds is helpful for the isolation of the intermediate β-hydroxysilanes, because magnesium strongly binds with oxygen, making the immediate elimination impossible. If excess organolithium or lithium amide base is used to generate the α-silyl carbanion, this base can effect the deprotonation as well, and since the lithium-oxygen bond is not as strong as magnesium-oxygen, the reaction leads directly to the alkene. Some reactions proceed with good diastereoselectivity, so the direct conversion can be an attractive option.
=====================================
Pinacol Coupling Reaction

This reaction involves the reductive homo-coupling of a carbonyl compound to produce a symmetrically substituted 1,2-diol. The first step is single electron transfer of the carbonyl bond, which generates radical ion intermediates that couple via carbon-carbon bond formation to give a 1,2-diol. The example depicted above shows the preparation of pinacol itself.
Pinacol and other highly substituted 1,2-diols tend to undergo dehydration with rearrangement under acid-catalysis (see Pinacol Rearrangement).
====================================
Pinacol Rearrangement

In the conversion that gave its name to this reaction, the acid-catalyzed elimination of water from pinacol gives t-butyl methyl ketone.
Mechanism of the Pinacol Rearrangement
This reaction occurs with a variety of fully substituted 1,2-diols, and can be understood to involve the formation of a carbenium ion intermediate that subsequently undergoes a rearrangement. The first generated intermediate, an α-hydroxycarbenium ion, rearranges through a 1,2-alkyl shift to produce the carbonyl compound. If two of the substituents form a ring, the Pinacol Rearrangement can constitute a ring-expansion or ring-contraction reaction.


====================================
Pinner Reaction


The Pinner Reaction is the partial solvolysis of a nitrile to yield an iminoether. Treatment of the nitrile with gaseous HCl in a mixture of anhydrous chloroform and an alcohol produces the imino ether hydrochloride. These salts are known as Pinner Salts, and may react further with various nucleophiles.
Mechanism of the Pinner Reaction





====================================
Prévost Reaction
The Prévost Reaction allows the synthesis of anti-diols from alkenes by the addition of iodine followed by nucleophilic displacement with benzoate in the absence of water. Hydrolysis of the intermediate diester gives the desired diol.
The Woodward Modification of the Prévost Reaction gives syn-diols.
Mechanism of the Prevost Reaction
The initial addition of iodine leads to a cyclic iodonium ion, which is opened through nucleophilic substitution by benzoate anion:A neighbouring-group participation mechanism prevents the immediate nucleophilic substitution of iodine by a second equivalent of benzoate that would lead to a syn-substituted product. Instead, a cyclic benzoxonium ion intermediate is formed:
Opening of this intermediate by a second addition of benzoate gives the anti-substituted dibenzoate:
Hydrolysis then delivers the diol.
In the Woodward-Modification, added water decomposes the above benzoxonium intermediate directly to a syn-substituted diol.
The use of expensive silver salts, the requirement for a stoichiometric amount of molecular halogen, and the formation of a relatively large amount of organic and inorganic wastes are definite drawbacks to this reaction. Sudalai recently reported on catalytic versions of both the Prévost Reaction and the Woodward-Modification.
L. Emmanuvel, T. M. A. Shaikh, A. Sudalai, Org. Lett., 2005, 7, 5071-5074.
The proper choice of stoichiometric oxidant allows either syn- or anti-selective dihydroxylations. NaIO4 as the oxidizing agent generates H2O as a side product of the oxidation and therefore enables the Woodward Reaction to take place.
High-valent iodine reagents are still relatively expensive, and the identification of a less costly stoichiometric oxidant would be a significant improvement for this process.
====================================
Prins Reaction
The Prins Reaction is the acid-catalyzed of addition aldehydes to alkenes, and gives different products depending on the reaction conditions. It can be thought of conceptually as the addition of the elements of the gem-diol carbonyl hydrate of the aldehyde across the double bond.
An excess of aldehyde and temperatures < 70 °C lead to the formation of acetals. When one equivalent of aldehyde is used and temperatures are > 70 °C diols or allylic alcohols may be isolated.


Although the mechanism is different, a Prins allylic alcohol product is equivalent to the result of an Ene Reaction.
Mechanism of the Prins Reaction




====================================
Pschorr Reaction

The Pschorr Reaction allows the preparation of biaryl tricyclics by intramolecular substitution of one arene by an aryl radical. This radical is generated in situ from an aryl diazonium salt by copper catalysis. Although excess copper salts are used, the yield is normally moderate.
Alternative one-electron donors that are more soluble have recently been discovered (see recent literature). The reported method leads to improved yields in a shorter reaction time.
Mechanism of the Pschorr Reaction

=====================================
CATAGORY R
Ramberg-Bäcklund Reaction
The Ramberg-Bäcklund Reaction allows a base-mediated conversion of α-halosulfones into E or Z alkenes. Z alkenes are often observed with weak bases, whereas strong bases give predominantly E alkenes. Myers' modification and newer synthetic protocols allow an in situ generation of α-halosulfones, so that the readily available sulfones can be used directly without performing a specific and more problematic halogenation step.
Mechanism of the Ramberg-Bäcklund Reaction
The Ramberg-Bäcklund Reaction is a convenient procedure for preparing alkenes, due to the accessibility of sulfones, the clearly defined location of the double bond as alkene rearrangements do not occur under basic conditions, and the broad range of substitution patterns that can be prepared including fully substituted alkenes, cyclobutenes and polyenes. Even polyfunctional molecules can be converted as long as base-sensitive groups are absent.The anionic mechanism for the Ramberg-Bäcklund Reaction is generally accepted. A rapid deprotonation is followed by a slow cyclization to generate a thiirane dioxide intermediate. In this step, α-iodosulfones react faster than bromo- and chlorosulfones.
Formation of the thiirane dioxide is followed by extrusion of SO2. Here, a concerted, linear cheletropic loss is symmetry forbidden, so the occurrence of dipolar and diradical stepwise mechanisms have been suggested. As the rate of reaction is influenced by the concentration of base, the formation of a hypervalent intermediate is generally accepted, but the question, whether the subsequent decomposition involves a rotationally hindered diradical or a concerted symmetry-allowed non-linear cheletropic pathway has not yet been answered.
The stereoselectivity for weak base and most substrates is well-defined, leading to Z alkenes. Moreover, cis-substituted thiirane dioxides, which have been prepared to elucidate the mechanism, selectively lead to Z alkenes if heated or treated with KOH.
In the reaction with KOH, the selectivity is already well-defined in the cyclization step. There is no epimerization during the formation of the alkene from a pure cis-subsituted thiirane dioxide. The formation of the cis-substituted thiirane dioxides seems to be favored, possibly due to diastereomeric carbanion formation, or attractive dispersion, or steric attraction. However, there is also an epimerization from the cis- to the trans-substituted thiirane dioxide when strong bases are used, as shown for the conversion of the cis-substituted thiirane dioxide. So the overall conversion can be summarized as:
As mentioned above, some substrates give E-substituted alkenes. For example, in α-chlorobenzyl benzyl sulfone, the presence of phenyl as acidifying substituent favors epimerization and leads to the energetically more stable trans-substituted thiirane dioxide:
The same diastereoselectivity can also be observed for the Myers' modification, exemplified by the conversion of dibenzyl sulfone:
However, a recent publication by Taylor shows unexpected Z-stereoselectivity in the Ramberg-Bäcklund reaction of diarylsulfones leading to cis-stilbenes, so the situation is somewhat more complicated for substituted arenes.
For the preparation of α-halosulfones, sulfides can be first chlorinated and then oxidized (first example), or the readily available sulfones can be used, in which an α-position can be deprotonated (for example using BuLi) and subsequently halogenated using an electrophilic halogenating reagent such NCS or NBS. The second example shows a convenient alternative using an alanate as intermediate:
Myers' modification circumvents this often problematic synthetic step by using an in situ halogenation-Ramberg-Bäcklund sequence in the presence of CCl4 as both solvent and electrophilic halogenating reagent. After halogenation by the solvent, the product undergoes cyclization and olefin formation as know from the unmodified Ramberg-Bäcklund reaction.
One drawback is the production of the highly reactive dichlorocarbene that can undergo addition to electron rich alkenes producing gem-dichlorocyclopropane adducts, which limits the synthetic scope.
====================================
Reformatsky Reaction

The formation of ester-stabilized organozinc reagents and their addition to carbonyl compounds
Mechanism of the Reformatsky Reaction
Organozinc compounds are prepared from α-halogenesters in the same manner as Grignard Reagents. This reaction is possible due to the stability of esters against organozincs. Due to the very low basicity of zinc enolates, there is hardly any competition from proton transfer, and the scope of carbonyl addition partners is quite broad. In presence of ketones or aldehydes, the organozinc compounds react as the nucleophilic partner in an addition to give β-hydroxy esters. An
ester-stabilized organozinc reagent
An
ester-stabilized organozinc reagentCompared to organolithiums and organomagnesium halides (Grignard reagents), the organozinc halide reagents used in the Reformatsky Reaction are relatively stable, and many are available commercially.
====================================
Ring Closing Metathesis (RCM)

The Ring-Closing Metathesis (RCM) allows synthesis of 5- up to 30-membered cyclic alkenes. The E/Z-selectivity depends on the ring strain.
The Ru-catalysts used tolerate a variety of functional groups, but normally the molecule must have polar side chains that are able to build a template for the catalyst. The modern Second Generation Grubb's Catalysts (see Olefin Metathesis) are more versatile.
Mechanism of Ring Closing Metathesis
The key intermediate is a metallacyclobutane, which can undergo cycloreversion either towards products or back to starting materials. When the olefins of the substrate are terminal, the driving force for RCM is the removal of ethene from the reaction mixture.Initiation:

Catalytic cycle:
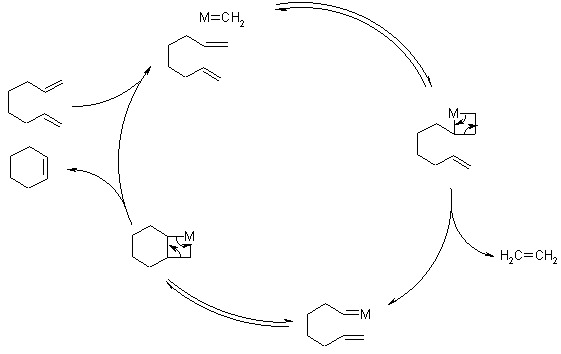 Chauvin's Mechanism
Chauvin's Mechanism====================================
Ring Opening Metathesis (Polymerization) - ROM(P)

Strained rings may be opened by a ruthenium carbene-catalyzed reaction with a second alkene following the mechanism of the Cross Metathesis. The driving force is the relief of ring strain. As the products contain terminal vinyl groups, further reactions of the Cross Metathesis variety may occur. Therefore, the reaction conditions (time, concentrations,...) must be optimized to favor the desired product.
Strain rings may be opened by a ruthenium carbene-catalyzed reaction with a second alkene following the mechanisms of the Cross Metathesis. Driving force is the relief of ring strain. As the products contain terminal vinyl groups, further reactions of the Cross Metathesis variety may occur. Therefore, the reaction conditions (time, concentrations,...) must be optimized to favor the desired product.
In absence of excess of a second reaction partner, polymerization occurs (ROMP):

The reverse reaction - the Ring Closing Metathesis - is a valuable synthesis tool for preparing from 5- up to 30-membered rings.
Mechanism of Ring Opening Metathesis
see Olefin Metathesis====================================
Ritter Reaction


The acid-induced nucleophilic addition of a nitrile to a carbenium ion, followed by hydrolysis to the corresponding amide.
Mechanism of the Ritter Reaction
Any substrate capable of generating a stable carbenium ion is a suitable starting material; primary alcohols do not react under these conditions, with exception of benzylic alcohols:
The carbenium ion adds to the nitrile nitrogen to give a nitrilium ion intermediate, which undergoes hydrolysis to the corresponding amide upon aqueous work-up.
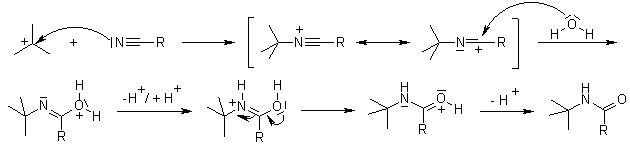
====================================
Robinson Annulation

The Robinson Annulation is a useful reaction for the formation of six-membered rings in polycyclic compounds, such as steroids. It combines two reactions: the Michael Addition and the Aldol Condensation
Mechanism of the Robinson Annulation
The first step in the process is the Michael Addition to an α,β-unsaturated ketone, such as methyl vinyl ketone:
The newly formed enolate intermediate must first tautomerize for the conversion to continue:

The subsequent cyclization via Aldol Addition is followed by a condensation to form a six-membered ring enone:

The Robinson Annulation can also proceed under acidic catalysis, with the entire process occurring in one pot, as shown below. The use of a precursor of the α,β-unsaturated ketone, such as a β-chloroketone, can reduce the steady-state concentration of enone and decrease the side reaction of polymerization.

a) C. H. Heathcock, J. E. Ellis, J. E. McMurry, A. Coppolino, Tetrahedron Lett., 1971, 12, 4995. DOI
b) C. H. Heathcock, C. Mahaim, M. F. Schlecht, T. Utawanit, J. Org. Chem., 1984, 49, 3264.
====================================
Rosenmund Reduction

The catalytic hydrogenation of acid chlorides allows the formation of aldehydes.
Mechanism of the Rosenmund Reduction
Side products:
The Pd catalyst must be poisoned, for example with BaSO4, because the untreated catalyst is too reactive and will give some overreduction. Some of the side products can be avoided if the reaction is conducted in strictly anhydrous solvents.
=====================================
Rosenmund-von Braun Reaction
Aryl nitriles can be prepared by the cyanation of aryl halides with an excess of copper(I) cyanide in a polar high-boiling solvent such as DMF, nitrobenzene, or pyridine at reflux temperature.
Mechanism of the Rosenmund-von Braun Reaction
The mechanism probably involves the formation of a Cu(III) species through oxidative addition of the aryl halide. Subsequent reductive elimination then leads to the product:The excess of copper cyanide and the use of a polar, high-boiling point solvent makes the purification of the products difficult. In addition, elevated temperatures (up to 200°C) lower the functional group tolerance. The use of alkali metal cyanides or cyanation reagents such as cyanohydrins, a catalytic amount of copper(I) iodide and kalium iodide, allows a mild, catalytic cyanation of various aryl bromides.

If aryl iodides, sodium cyanide and copper(I) iodide are used, a simple mechanism similar to that of an Ullmann-type reaction can be proposed:
Reactions with aryl bromides and added alkali metal iodides involve additional equilibria in which aryl bromides give the more reactive aryl iodides:
For example, the formation of a copper(III) species and the use of cyanohydrins is discussed by H.-J. Christeau (Chem. Eur. J., 2005, 11, 2483. DOI).
====================================
Rubottom Oxidation

The synthesis of α-hydroxy ketones is achieved by reaction of silyl enol ethers with mCPBA, with subsequent rearrangement. Aqueous work up or reaction with TBAF (fluoride ions) gives the desired product after desilylation
Mechanism of the Rubottom Oxidation
The enol ether double bond is epoxidized by the peracid. Relief of the epoxide ring strain drives the rearrangement with migration of the silyl group to give the silylated α-hydroxy ketone product.
========================================================================
No comments:
Post a Comment